Microbiological assays use microorganisms to ascertain the potency of medications. There's two main solutions - the cylinder-plate process which actions inhibition zone diameters, as well as turbidimetric strategy which measures absorbance adjustments in liquid cultures.
Prepare personnel on the significance of remaining knowledgeable about updates to testing procedures, regulatory requirements, and sector finest practices. Stimulate participation in education classes and workshops to facilitate ongoing Mastering.
This growth brings about strains that happen to be tougher to manage, complicating attempts to ascertain productive microbial limits.
In summary, knowledge microbial limits is vital for shielding general public health and fitness. These limits directly affect water basic safety and foodstuff regulation, addressing considerable overall health fears around the world. By adhering to proven requirements, we could avert outbreaks and safeguard Local community well being.
Give an extensive introduction to the principles and importance of Microbial Limit Testing. This involves comprehending the importance of testing Uncooked products and finished products for microbial contamination.
Deviations pose a threat to solution high-quality, possibly resulting in non-compliance with regulatory standards. Addressing deviations instantly is critical to stop compromised product or service safety and secure the track record on the Corporation.
* Use deviations as possibilities for continual advancement. Carry out reviews and conversations to determine lessons discovered from Each and every deviation, facilitating ongoing enhancement of Microbial Limit Testing procedures.
If no colonies are noticed Categorical the result as a number of colonies lower than dilution component. Observe down the result.
Adequately defining and checking these limits is essential for making certain protection and good quality throughout numerous fields, particularly in pharmaceuticals and food items production.
Collaboration also prepares long term scientists, guaranteeing the continual advancement of microbial analysis.
Pinpointing microbial limits provides sizeable difficulties that have to be resolved to be sure general public health and basic safety. These worries crop up from various elements, together with environmental variability along with the rising issue of website antimicrobial resistance.
The precise facet of ISO standards pertinent below features their comprehensive framework for chance administration. This permits corporations to undertake most effective tactics for microbial Manage effectively and properly.
Foodstuff protection is an additional crucial space motivated by microbial limits. Microbial contamination can occur at any position from farm to table. Polices governing foodstuff safety intention to minimize challenges linked get more info to unsafe microorganisms in meals products.
Once microbial testing is total, the QC Office is to blame for analyzing and interpreting the information. This consists of evaluating the obtained microbial counts with recognized acceptance criteria.
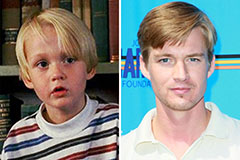 Mason Gamble Then & Now!
Mason Gamble Then & Now! Jeremy Miller Then & Now!
Jeremy Miller Then & Now! Tiffany Trump Then & Now!
Tiffany Trump Then & Now! Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Ricky Schroder Then & Now!
Ricky Schroder Then & Now!